About our group
Radioactive labels used for tracing of studied ligands have long been a part of the biochemical laboratory repertoire. Radioactivity gives a clear, unmistakable signal, and its use is fairly straightforward. We are devoted to supply radioactively labeled compounds to biochemical research teams of the institute, provide radiometric services, conduct radioactive waste management and supervise radiation safety rules within the institute.
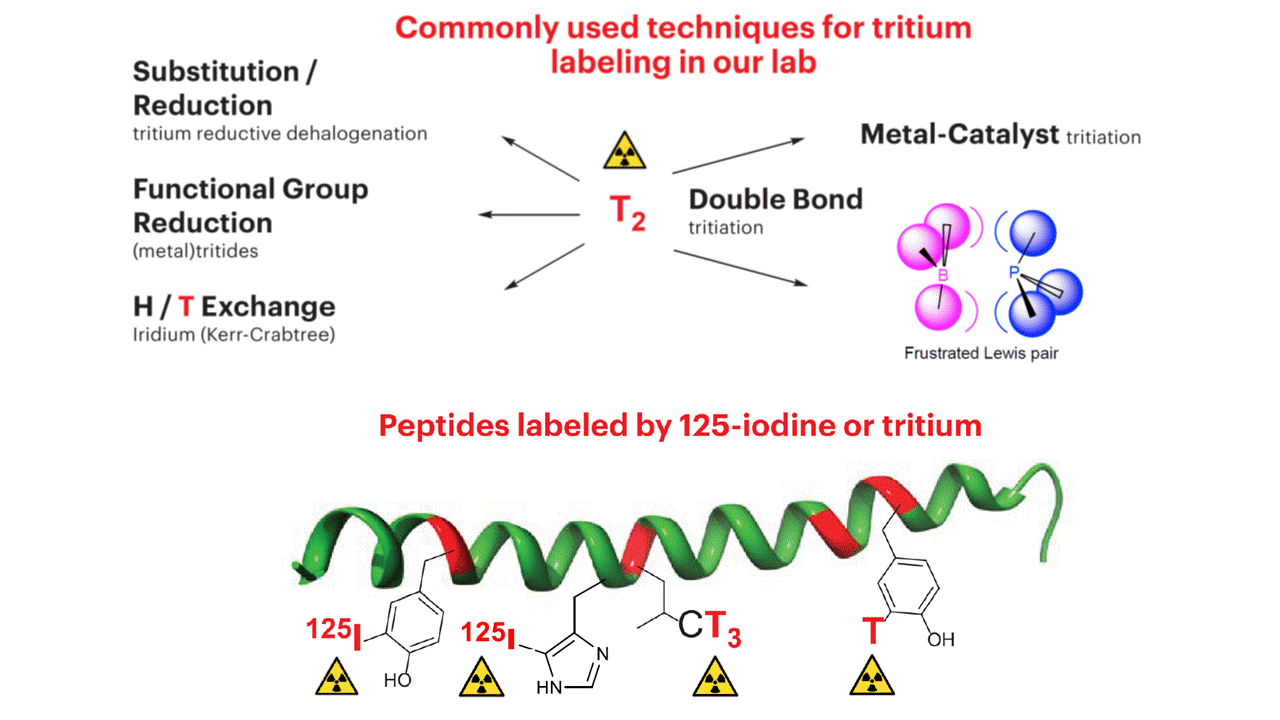
GAMMA RAY EMITTER - 125I
We use optimized low-cost labeling procedure of peptides/polypeptides with IODO–GEN™– Na[125I] system. Produced are purified mono-iodinated peptides with specific activities over 2 000 Ci/mmol, separated from over-iodinated and starting peptides using radio-HPLC. Lyophilized 125I-labeled peptides can be stored at -20 °C for a long period of time.
β-PARTICLE EMITTER - 3H (14C)
Tritium/carbon-14 labeling of complex, multifunctional drug candidates requires mild, fast and safe preparative methods. We have expertise handling well-established methods used for introduction of tritium into biologically active molecules – from simple catalytic hydrogen isotope exchange through reduction of carbon-carbon multiple bonds, catalytic reductive dehalogenations to reductions with in-house synthesized carrier-free complex metallic tritides. We have been investigating the feasibility of Frustrated Lewis Pairs (FLP) as a mild catalysts for various tritiations in the quest for alternative orthogonal tritiation methods. The analysis and radiochemical purity of produced tracers is always checked by radio-HPLC, LC-MS, GC-MS, radio-TLC and 3H NMR spectra.
News
Show allPublications
All publications